E-Clinical Solution Market Size, Share, Development, Growth and Demand Forecast to 2020 – Industry Insights by Type (Products, Professional Services), by Products (CTMS, EDC/ CDMS, Safety Solution, eCOA Solution, RTSM Solution), by Delivery Mode (Web based E-Clinical Solution, On-Premise E-Clinical Solution, Cloud based E-Clinical Solution) by End Users (Healthcare Providers, Pharmaceutical and Biotechnology Companies, Clinical Research Organizations)
The global eClinical solutions market is estimated to reach 7.61 Billion by 2022, at a CAGR of 12.4% during the forecast period (2017-2022).
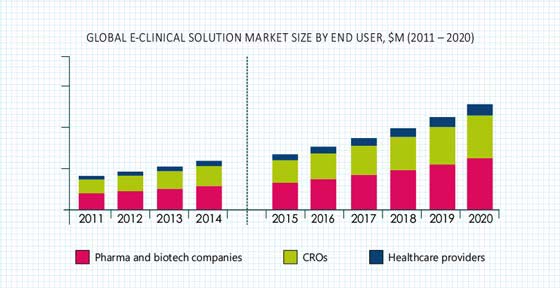
The adoption of novel software solutions during clinical research and rising government funding to support clinical trials are key factors fueling the growth of the market. Furthermore, increased outsourcing of clinical trial processes by industrial researchers to contract research organizations (CROs) offers growth opportunities for market players.
The electronic data capture (EDC) and clinical data management system (CDMS) segment accounted for the largest share of the global eClinical solutions market in 2016. The electronic clinical outcome assessment (eCOA) segment is expected to achieve the highest CAGR during the forecast period. The increasing demand for eDiaries in clinical studies to collect patient self-reports by major pharmaceutical companies and the use of mobile and digital technologies are driving the growth of this market segment.
Emerging markets including China, South Korea, Taiwan, and India are attractive destinations for outsourcing clinical trials due to the presence of a large patient population and the low operating cost of conducting clinical trials in these countries. Less stringent regulatory guidelines compared to developed nations, shortage of trial volunteers in Europe and North America, and the growing number of life science research companies and CROs are key factors propelling the demand for eClinical solutions in these emerging markets. In order to leverage the high growth opportunities, leading market players are expanding their presence in these nations.
Geographically, the growth of the North American market is getting driven due to the increasing need for standardization of data and increase in spends on research and development by pharmaceutical and biotechnology companies. The U.S. accounted for the largest share in the North American e-clinical solution market. According to the U.S. National Institutes of Health (Clinical Trial government registry), about 89,528 clinical trials were conducted in the U.S., as of October 2015.
In Europe, according to eClinical Visions, a publication from Oracle Health Sciences, the percentage of clinical trials in Western Europe decreased from 25% in 2006 to 19% in 2010. Germany accounted for the largest share in the European market. However, the Asia-Pacific market is expected to witness the highest growth during the forecast period. This is mainly due to growing clinical trial outsourcing, easy patient recruitment, large pool of patient, and increasing prevalence of chronic and lifestyle associated diseases in the region.
As of 2016, Oracle Corporation (U.S.) held the leadership position in the global eClinical solutions market. Over the past three years, the company has adopted partnerships, agreements, and collaborations and product launches, enhancements, and deployments as key business strategies to ensure its dominance. Medidata Solutions, Inc. (U.S.) PAREXEL International Corporation (U.S.), BioClinica, Inc. (U.S.), CRF Health (U.S.), DATATRAK International, Inc. (U.S.), ERT (U.S.), MaxisIT Inc. (U.S.), Bio-Optronics, Inc. (U.S.), eClinical Solutions, Inc. (U.S.), Merge Healthcare Incorporated (U.S.), and OmniComm Systems, Inc. (U.S.) are some of the other key players in this market.
Target Audience
- Healthcare IT Service Providers
- eClinical Solution Vendors
- Clinical Research Organizations
- Pharmaceutical/Biopharmaceutical Companies
- Research and Development (R&D) Companies
- Business Research and Consulting Service Providers
- Medical Research Laboratories
- Academic Medical Centers/Universities/Hospitals
To know about the assumptions considered for the study, download the pdf brochure
Scope of the Report
This report categorizes the eClinical solutions market into the following segments:
Global eClinical Solutions Market, by Product
- Electronic Data Capture (EDC) and Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Clinical Analytics Platform
- Randomization and Trial Supply Management (RTMS)
- Clinical Data Integration Platform
- Electronic Clinical Outcome Assessment (eCOA)
- Safety Solutions
- Electronic Trail Master Files (eTMF)
- Regulatory Information Management Solutions (RIMS)
- Others (Coding Systems, Institutional Review Board Systems, and Core Lab Integration Solutions)
Global eClinical Solutions Market, by Delivery Mode
- Web-hosted (On-demand)
- Licensed Enterprise (On-premise)
- Cloud-based (SaaS)
Global eClinical Solutions Market, by Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Global eClinical Solutions Market, by End User
- Pharmaceutical and Biopharmaceutical Companies
- Contract Research Organizations
- Consulting Service Companies
- Medical Device Manufacturers
- Hospitals
- Academic Research Institutions
Global eClinical Solutions Market, by Region
- North America
- U.S.
- Canada
- Europe
- Asia-Pacific
- Rest of the World
Available Customizations
With the given market data, MarketsandMarkets offers customizations as per the company’s specific needs. The following customization options are available for the report:
Product Analysis
- Product Matrix, which gives a detailed comparison of the product portfolios of the top five companies in the eClinical solutions market
Contract Research Organizations (CROs) Market Segmentation, by Clinical Trial Phase
- Contract research organizations (CROs) market segmentation by clinical trial phase (including Phase I, Phase II, Phase III, and Phase IV clinical trials)
Company Information
- Detailed analysis and profiling of additional market players (up to five)

.jpg)

Comments
Post a Comment